Pharma doctor engagement automation 2026: Capitalizing on Union Budget healthcare opportunities with AI-personalized video at scale
Estimated reading time: ~12 minutes
Key Takeaways
- Union Budget 2026–27 unlocks major healthcare opportunities for pharma/medtech, demanding digital-first, compliant HCP outreach.
- AI-personalized video enables scalable, multilingual, compliance-vetted education across CME, policy updates, device launches, and trials.
- HCP engagement automation improves message recall, governance (UCPMP/UCMPMD), and auditability across 1.3M+ practitioners.
- A 30–60–90 day plan operationalizes pilots, omnichannel delivery (e.g., WhatsApp), and dashboards to measure ROI.
- Ethics and compliance require fair balance, clear sponsorship, opt-in data practices, and immutable audit trails.
The unveiling of the Union Budget 2026–27 has signaled a transformative era for the Indian healthcare landscape, creating unprecedented Union Budget healthcare opportunities for pharmaceutical and medtech organizations. With India’s healthcare sector now prioritizing digital infrastructure and regional medical hubs, the race to engage the nation’s ~1.3 million registered practitioners has intensified. In this high-stakes environment, pharma doctor engagement automation 2026 has emerged as the definitive pathway for brands to deliver compliant, personalized, and scientifically rigorous content at a scale previously deemed impossible. By leveraging healthcare professional video marketing, life sciences leaders can now bridge the gap between policy shifts and clinical practice, ensuring that every physician receives the right information at the right time.
Capitalizing on Union Budget Healthcare Opportunities for Pharma and MedTech
The Union Budget 2026–27 has allocated significant resources toward the Ministry of Health and Family Welfare (MoHFW) and the National Health Mission (NHM), with a specific focus on the Ayushman Bharat Digital Mission (ABDM) and the Pradhan Mantri Ayushman Bharat Health Infrastructure Mission (PM-ABHIM). These Union Budget healthcare opportunities are not merely fiscal allocations; they are blueprints for how pharma and medtech companies must align their outreach. For instance, the push for ABDM enablement means that doctors are increasingly operating within a digital-first framework, requiring healthcare policy update videos that explain new digital standards, e-prescription protocols, and interoperability in real-time.
Furthermore, the budget’s emphasis on primary and secondary care strengthening through PM-ABHIM necessitates a massive surge in continuing medical education videos. As the government scales public health capacity to manage non-communicable diseases (NCDs) and infectious disease modules, pharma companies have a unique window to provide localized, high-quality educational content. This alignment ensures that corporate social responsibility (CSR) and medical affairs initiatives are not just compliant but are actively contributing to national health priorities.
The 2026 budget also highlights a renewed focus on health research and ICMR-linked initiatives. This has direct implications for clinical trial recruitment videos, as the government seeks to position India as a global hub for clinical excellence. By automating the delivery of protocol explainers and investigator training modules, pharma companies can accelerate site activation and patient enrollment while maintaining the highest ethical standards.
Sources:
The Strategic Shift: Why Pharma B2B Doctor Engagement 2026 Requires Automation
In 2026, the traditional model of pharmaceutical sales—reliant heavily on physical field visits and static brochures—is no longer sufficient to capture the attention of a digitally fatigued medical community. Pharma B2B doctor engagement 2026 is defined by a shift toward omnichannel, data-driven interactions where AI-personalized video serves as the primary medium for scientific exchange. According to recent industry insights, 2026 will see hybrid roles and AI-integrated clinical workflows become the standard, forcing medical and commercial teams to retool for HCP engagement automation.
The necessity for automation stems from the sheer scale of the Indian market. Reaching 1.3 million Healthcare Professionals (HCPs) across diverse geographies and languages requires a system that can personalize content without manual intervention. Healthcare professional video marketing allows for superior message recall compared to text-based emails or PDFs. When a doctor receives a video that addresses them by name, references their specific specialty, and provides updates relevant to their city’s healthcare infrastructure, the engagement rate skyrockets.
Moreover, the regulatory environment in India has become more stringent with the full implementation of UCPMP 2024. Automation ensures that every touchpoint is logged, every claim is vetted, and every interaction is opt-in and transparent. This level of governance is impossible to maintain at scale without a robust technological backbone. Platforms like TrueFan AI enable pharmaceutical companies to generate thousands of these personalized, compliant videos in minutes, ensuring that the field force is supported by high-impact digital assets that reinforce their in-person discussions.
Sources:
The AI Video Layer: Transforming Healthcare Professional Video Marketing
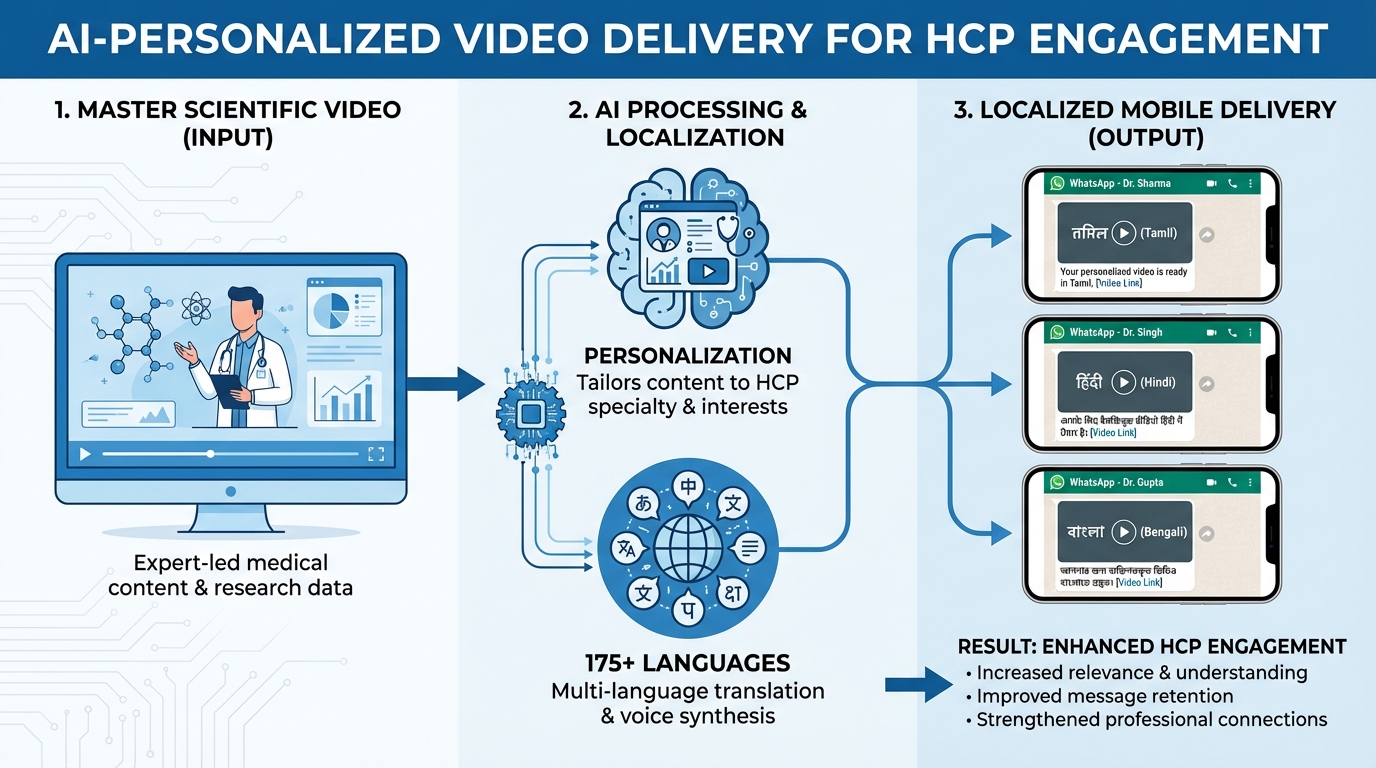
The core of modern healthcare professional video marketing lies in its ability to deliver scientific, compliance-vetted communication tailored to an HCP’s specific journey. This journey typically begins with awareness, where short scientific explainers or healthcare policy update videos introduce a new therapy or regulatory change. As the HCP moves toward deep-dive education, the content shifts to pharmaceutical education videos covering Mechanism of Action (MOA), indication, and dosage titration.
To be effective in 2026, these videos must adhere to specific technical and localized standards. Localization is no longer just about translation; it is about cultural and clinical relevance. TrueFan AI's 175+ language support and Personalised Celebrity Videos allow brands to reach doctors in their preferred tongue—whether it be Tamil, Telugu, Marathi, or Bengali—while maintaining the integrity of complex medical terminology. This level of personalization ensures that the doctor feels seen and respected as an individual practitioner.
The delivery of this content is equally critical. In the Indian context, WhatsApp Business API has become the preferred channel for HCPs, provided the content is high-value and non-intrusive. By embedding personalized videos into WhatsApp templates or secure microsites with UTM tagging, pharma companies can track exactly how much of the video was watched and whether the HCP engaged with the call-to-action (CTA). This data then flows back into the CRM, allowing for a seamless transition from digital engagement to a rep-led discussion or a medical conference follow-up automation sequence.
Sources:
- TrueFan Enterprise capabilities and blog intelligence: Internal Product Documentation 2025–2026
- Department of Pharmaceuticals: UCPMP 2024
The HCP Engagement Automation Playbook: From CME to Rep Enablement
A successful HCP engagement automation strategy is built on event-driven triggers that ensure relevance. For instance, a new policy announcement or a label change should immediately trigger a healthcare policy update video sent to all relevant specialists. This not only positions the pharma company as a proactive partner but also ensures that the doctor is always practicing with the latest information. Similarly, the moment a medical representative completes a call, an automated, personalized recap video can be sent to the HCP, reinforcing the three key takeaways from the discussion.
One of the most potent applications of automation is in continuing medical education videos. In 2026, CME has evolved into laddered micro-learning units. An automated workflow can track a doctor’s progress through a series of 6–10 minute modules, sending reminders for incomplete units and automatically delivering a printable certificate upon completion of the assessment. This “micro-learning” approach respects the doctor’s time while ensuring high knowledge retention.
Furthermore, medical rep enablement videos are revolutionizing field productivity. Instead of generic training, reps now receive bite-sized, localized videos on their SFA (Sales Force Automation) apps that help them handle specific regional objections or refresh their knowledge on a new product launch. These videos can even be territory-specific, highlighting local formulary wins or regional clinical data. By empowering the field force with these tools, pharma companies ensure that the “human” element of engagement is as data-driven and effective as the digital one.
Sources:
- PRS India: Budget Analysis (Health) 2026–27
- TrueFan AI Cipla Case Study: Internal Enterprise Records
High-Impact Applications: Medical Device Launches and Clinical Trial Recruitment
The medtech sector in India is witnessing a “Make in India” surge, making medical device launch campaigns more competitive than ever. A personalized video-led launch framework allows medtech firms to engage KOLs (Key Opinion Leaders) during the pre-approval phase with indication-specific explainers. At the launch stage, personalized demos can be sent to surgeons and hospital administrators, highlighting the specific benefits of the device for their patient demographic. Post-launch, automation can be used to deliver adherence and usage best practices, reducing the risk of user error and improving patient outcomes.
In the realm of clinical research, clinical trial recruitment videos are solving the perennial challenge of investigator engagement and patient enrollment. For B2B investigator outreach, automated videos can explain complex protocol criteria and site setup checklists, ensuring that site PIs (Principal Investigators) are fully aligned with the study's goals. For patient-facing recruitment, these videos—once approved by the IRB/IEC—can be localized to ensure that potential participants fully understand the consent process in their native language, thereby improving the ethical transparency of the trial.
All device and trial-related content must strictly align with the Uniform Code for Marketing Practices in Medical Devices (UCMPMD 2024). This includes ensuring that all “Instructions for Use” (IFU) references are accurate and that fair-balance panels are prominently displayed. By automating the inclusion of these compliance elements into every video, companies can mitigate the risk of regulatory non-compliance while maintaining a rapid pace of communication.
Sources:
- Department of Pharmaceuticals: UCMPMD 2024 (Medical Devices)
- Fortune Business Insights: Medical Devices Market 2026
Navigating Ethics and Compliance: UCPMP and Prescription Influence Marketing
In the 2026 regulatory climate, prescription influence marketing must be approached with extreme caution and ethical rigor. The goal is no longer to “induce” prescriptions but to provide evidence-based, balanced education that improves appropriate use. This means that every pharmaceutical education video must include a clear disclosure of sponsorship, a fair balance of benefits and risks, and easily accessible links to the full prescribing information.
Pharma compliance marketing India requires a robust governance framework where every personalized video is part of an immutable audit trail. This includes capturing explicit opt-in consent, recording the timestamp of every interaction, and ensuring that content moderation filters block any unapproved or promotional-leaning language. Solutions like TrueFan AI demonstrate ROI through their ability to maintain these high standards of security and compliance (ISO 27001, SOC 2) while delivering hyper-personalized content at scale.
Furthermore, doctor loyalty programs in 2026 have been reimagined as value-based education hubs. Instead of monetary incentives, loyalty is built through exclusive access to advanced CME tracks, early insights into healthcare policy update videos, and clinical toolkits that help doctors manage their practice more effectively. This “value exchange” model is fully compliant with UCPMP 2024, as it focuses on professional development and patient care rather than personal benefit for the practitioner.
Sources:
Measuring ROI and Scaling with TrueFan AI’s Enterprise Platform
The ultimate validation of pharma doctor engagement automation 2026 lies in its measurable impact on business and clinical outcomes. A comprehensive ROI framework CFO playbook for Union Budget 2026 marketing opportunities must look beyond simple “views.” It should track engagement depth (video completion rates), knowledge uplift (quiz scores within CME modules), and behavioral shifts (clicks to request a medical information query or book a rep meeting). By segmenting this data by specialty, city, and language, pharma leaders can gain a granular understanding of what resonates with their audience.
TrueFan AI’s Enterprise platform is designed to execute this end-to-end journey. A notable proof point is the Cipla Doctors’ Day activation, where 6,400 personalized videos were delivered to doctors, resulting in an overwhelmingly positive response and strengthened B2B relationships. The platform’s ability to render personalized videos in under 30 seconds and integrate directly with existing CRM/SFA stacks makes it an ideal partner for large-scale pharmaceutical operations.
For organizations looking to begin this journey, a 30-60-90 day execution plan is recommended:
- 0–30 Days: Define compliance guardrails, select a pilot therapy area, and integrate the API with your CRM/WhatsApp Business account.
- 31–60 Days: Launch the first set of healthcare policy update videos and medical conference follow-up automation drips. Calibrate the multilingual QA process.
- 61–90 Days: Expand to medical device launch campaigns or clinical trial recruitment videos. Publish a comprehensive ROI dashboard to stakeholders.
Conclusion
The convergence of the Union Budget 2026 healthcare priorities and the rise of generative AI has created a “perfect storm” for pharmaceutical and medtech marketing. Organizations that embrace pharma doctor engagement automation 2026 will not only achieve greater reach among India’s 1.3 million HCPs but will also build deeper, more meaningful relationships based on value, education, and trust. The window to capitalize on these Union Budget healthcare opportunities is now. By integrating healthcare professional video marketing into the core of your commercial and medical strategy, you can ensure that your brand remains at the forefront of the digital health revolution in India.
Ready to transform your HCP engagement? Book an enterprise demo with TrueFan AI today to explore how hyper-personalized video can scale your 2026 medical and commercial initiatives.
Recommended Internal Links
- Healthcare professional video marketing in India
- HCP engagement automation: Smarter pharma strategies 2026
- Medical device launch campaigns
- Union Budget 2026 sector opportunities
- Union Budget 2026 marketing strategies (Guide)
- Union Budget 2026 marketing opportunities (CFO Playbook)
Frequently Asked Questions
How to implement pharma doctor engagement automation 2026 without breaching UCPMP?
Implementation must focus on scientific education and professional development. Ensure all videos include fair balance, sponsorship disclosures, and are delivered only to HCPs who have provided explicit opt-in consent. Platforms like TrueFan AI provide the moderation and audit trails necessary to ensure every video remains within these ethical boundaries.
Are personalized healthcare professional video marketing campaigns compliant in India?
Yes, provided they adhere to UCPMP 2024 and (for devices) UCMPMD 2024. The personalization must be used to enhance the relevance of scientific information (e.g., addressing the doctor by name and specialty) rather than offering any form of inducement or gift.
What metrics prove ROI for HCP engagement automation?
Key metrics include the Video Completion Rate (VCR), knowledge retention scores from embedded quizzes, the number of rep appointments booked via video CTAs, and the cost-efficiency of reaching 1.3M HCPs compared to traditional field-only models.
How do medical device launch campaigns benefit from personalized video in India?
Personalized video allows for specialty-specific demos, localized safety training, and direct links to regional distributors. This ensures that surgeons and hospital staff receive information tailored to their specific clinical environment and language preference.
Can AI videos be updated if a policy or label changes?
Yes. Advanced AI platforms allow for “virtual reshoots,” where specific lines of dialogue or on-screen text can be updated across thousands of existing videos without the need for a new production cycle. This is particularly useful for rapid responses to Union Budget updates or NPPA price notifications.




